Dielectrics or Insulators
A dielectric is a non-conducting material and has no free electrons. The electrons in a dielectric are bound within the atoms. Ebonite, glass and mica are some examples of dielectrics. When an external electric field is applied, the electrons are not free to move anywhere but they are realigned in a specific way.
In dielectrics, all the electrons are bound to their parent molecules and there are no free charges. Even with normal voltage or thermal energy, electrons are not released.
A dielectric is made up of either polar molecules or non-polar molecules.
Non-polar molecules
A non-polar molecule is one in which centers of positive and negative charges coincide. As a result, it has no permanent dipole moment. Examples of non-polar molecules are hydrogen (H2), oxygen (O2), and carbon dioxide (CO2) etc.
When an external electric field is applied, the centers of positive and negative charges are separated by a small distance which induces dipole moment in the direction of the external electric field. Then the dielectric is said to be polarized by an external electric field. This is shown in Figure.
Polar molecules
In polar molecules, the centers of the positive and negative charges are separated even in the absence of an external electric field. They have a permanent dipole moment. Due to thermal motion, the direction of each dipole moment is oriented randomly (Figure (a)). Hence the net dipole moment is zero in the absence of an external electric field. Examples of polar molecules are H2O, N2O, HCl, NH3.
When an external electric field is applied, the dipoles inside the polar molecule tend to align in the direction of the electric field. Hence a net dipole moment is induced in it. Then the dielectric is said to be polarized by an external electric field (Figure (b)).
Polarisation
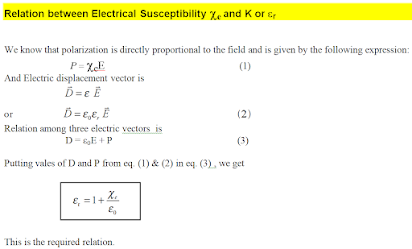
In the presence of an external electric field, the dipole moment is induced in the dielectric material. Polarisation P is defined as the total dipole moment per unit volume of the dielectric. For most dielectrics (linear isotropic), the Polarisation is directly proportional to the strength of the external electric field. This is written as
where χe is a constant called the electric susceptibility which is a characteristic of each dielectric.
Induced Electric field inside the dielectric
When an external electric field is applied on a conductor, the charges are aligned in such a way that an internal electric field is created which cancels the external electric field. But in the case of a dielectric, which has no free electrons, the external electric field only realigns the charges so that an internal electric field is produced. The magnitude of the internal electric field is smaller than that of external electric field. Therefore the net electric field inside the dielectric is not zero but is parallel to an external electric field with magnitude less than that of the external electric field. For example, let us consider a rectangular dielectric slab placed between two oppositely charged plates (capacitor) as shown in the Figure 1.52(b).
The uniform electric field between the plates acts as an external electric field ext which polarizes the dielectric placed between plates. The positive charges are induced on one side surface and negative charges are induced on the other side of surface.
But inside the dielectric, the net charge is zero even in a small volume. So the dielectric in the external field is equivalent to two oppositely charged sheets with the surface charge densities +σb and –σb. These charges are called bound charges. They are not free to move like free electrons in conductors. This is shown in the Figure (b).
For example, the charged balloon after rubbing sticks onto a wall. The reason is that the negatively charged balloon is brought near the wall, it polarizes opposite charges on the surface of the wall, which attracts the balloon. This is shown in Figure 1.53.
Dielectric strength
When the external electric field applied to a dielectric is very large, it tears the atoms apart so that the bound charges become free charges. Then the dielectric starts to conduct electricity. This is called dielectric breakdown. The maximum electric field the dielectric can withstand before it breakdowns is called dielectric strength. For example, the dielectric strength of air is 3 × 106 V m-1. If the applied electric field increases beyond this, a spark is produced in the air. The dielectric strengths of some dielectrics are given in the Table.
Dielectric Breakdown
The dielectric breakdown is the sudden change in state of a dielectric material subjected to a very high electric field , under the influence of which , the electrons are lifted into the conduction band causing a surge of current , and the ability of the material to resist the current flow suffers a breakdown .
Dielectric breakdown is of two types :
1.Thermal breakdown
2.Discharge breakdown
3.Defect breakdown
4.Intrinsic breakdown
5. Electro Chemical breakdown
Dielectric constant or relative permittivity
Dielectric constant is the ratio of the capacitance of the capacitor filled with dielectric material to the capacitance of the capacitor with air medium for the same capacitor.
It is defined as the ratio of the permittivity of the given medium to the permittivity of free space.
Short Questions and Answers
1. What do you understand by dielectrics?
Answer: Dielectrics are non-conducting materials like insulators.
2. What is the difference between dielectrics and insulators?
Answer: There is a slight difference between dielectrics and insulators which is the function they perform.
The main function of a dielectric material is to store electric energy in the form of potential energy while the function
of an insulator is to obstruct of flow of current.
3. What is dielectric constant?
Answer: Dielectric constant is the ratio of the capacitance of the capacitor filled with dielectric
material to the capacitance of the capacitor with air medium for the same capacitor.
4. What do you understand by polarization?
Answer: Induced dipole moment per unit volume is called polarization and the materials are said to be polarized.
5. What do you understand by polar and nonpolar molecules?
Answer: When centers of gravity of positive and negative charges coincide, the molecule is said to be non-polar. The non-polar molecules have zero permanent dipole moment due to symmetrical structure.
Some example are H2, N2, Cl2, O2, CCl4, BF3, CO2, etc.
When the centers of gravity of positive and negative charges do not coincide, the molecule is said to be polar. The
polar molecules have permanent dipole moment due to non-symmetrical structure. Some examples
are H2O, HCl, NH3, CH3Cl, CO, etc.
6. Define electric dipole and electric dipole moment?
Answer: A pair of equal and opposite charges separated by a small distance is known as an electric dipole. However the product of magnitude of one of the charges and the distance between the two charges is called the dipole
moment.
7. What is internal field in dielectric?
Answer: Internal field or local field is the sum of external field and the field generated by dielectric molecules.
8. What is dielectric loss?
Answer: The amount of energy dissipated in the form of heat by a dielectric medium under the action of external electric field is known as dielectric loss.

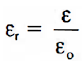

Nice
ReplyDeleteBest
ReplyDeleteTo the point
ReplyDelete